knowledge
Read more
If you want to know what’s on our minds or get inspired …

Quantify to Qualify
Get tools for quantifying user
input for drug delivery systems
enabling sound choices in the
development process.

How an FDA pre-submission enhances your usability engineering process
Discover the advantages of incorporating FDA pre-submission insights into your usability engineering process for streamlined success.
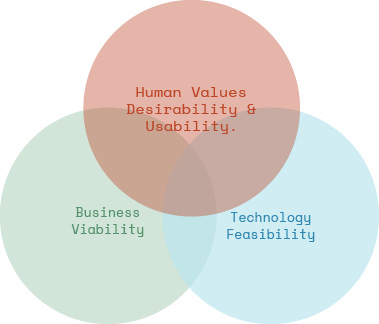
How not to be part of the 80% of product innovations that fail
It’s not just about having a great idea but also about balancing desirability, feasibility, and viability.

Data and imagination in innovation
In this article, we’ll explore why imagination is just as important as analytics when it comes to driving innovation.

Bridging drug and device
Read this whitepaper on how to bridge the gap between drug and device on end-to-end device development projects and device platform assessment tasks.
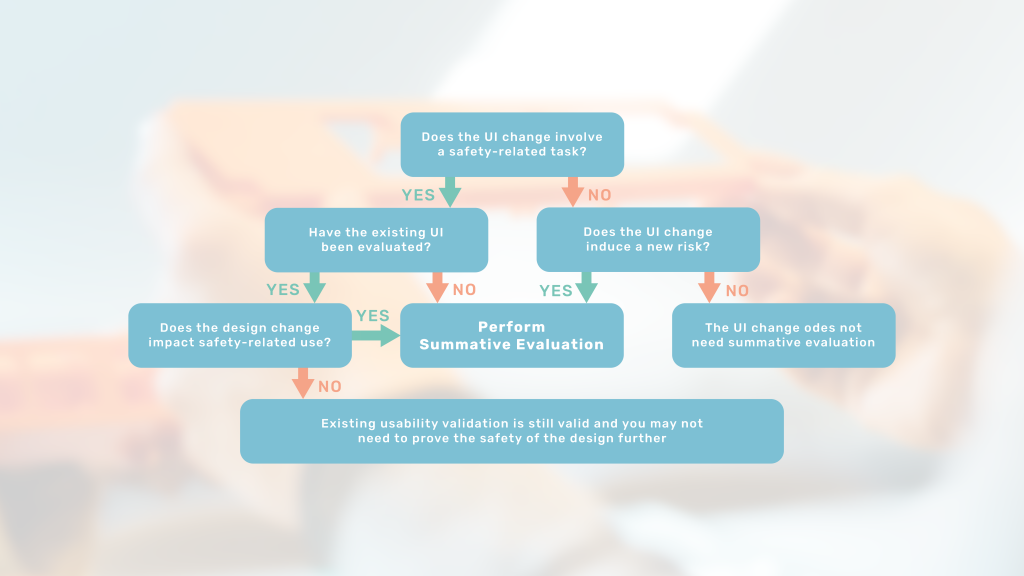
When is a new summative evaluation needed?
This article will introduce you to assessing the usability impact of your design change and thereby plan for any needed summative evaluation.

Using task analysis to identify potential use errors and get valuable design input
Find out how you can identify use errors and their sources and get valuable design input with PCA analysis.

What are the Benefits of Simulation with the Finite Element Method?
Will a medical device break apart when dropped onto a concrete floor? Does the device remain safe and functional if stored for years on a shelf before reaching the patient?

Why you should apply virtual DoE to the design process
Early product insights and fewer physical tests are some of the benefits of using Design of Experiments in medical device development.

Medical device regulations: obstacle or golden opportunity?
Healthtech magazine article shines the spotlight on the challenges and rewards of building your business processes around regulatory compliance.

So you want to launch a product people will love?
You have an idea for a new product. It is actually great and you think to yourself: ‘If I find this useful surely others will, too. People even say they can’t wait to get their hands on it.

Design Control as a discipline
Technolution’s design control specialists can quickly gain an overview of the product and the project to ensure a red thread during the design control documentation process.

A checklist for better product requirements
Too many projects stall out or fail due to ambiguous or unspecific design input requirements. We give you a checklist for writing requirements that won’t be misinterpreted.

The highlights of the updated risk management standard
What you need to know about the updates to the 2019 version of ISO 14971.

Implementing an effective Risk Management process
Make the Risk Management process work for you with a few tips for smarter documents and procedures.