Simulating functionality
What are the benefits of Simulation with the Finite Element Method?
Developing new medical devices can be time consuming and costly. In this article, Technolution explains the advantages of using software simulation tools based on the Finite Element Method to reduce development time and cost.
Will a medical device break apart when dropped onto a concrete floor? Does the device remain safe and functional if stored for years on a shelf before reaching the patient?
These are some of the important questions during the development of new medical devices that can be answered digitally with simulation software based on the Finite Element Method (FEM). FEM makes early concept decisions easier and reduces the need for making physical prototypes. It also makes it possible to test performance and behavior of a device that simply cannot be done in the real world.
– “In a sense, you are able to experiment with product prototypes for free. It becomes much easier to narrow down the best concepts for further investigation, thereby shortening time-to-market and improving innovation,” says Alexandre Gegelashvili, Senior R&D Engineer at Technolution.

A virtual twin
Simply put, FEM is a set of numerical mathematical methods implemented in a software. One can think of the software as a digital workbench for creating and assessing many different versions of a product concept and their properties without having to manufacture every single one of them physically as prototypes. A CAD model of the product is converted into a finite number of elements, thereby becoming a simulation model for the computer to perform calculations on – like a virtual twin of a real-world prototype that the engineers can tinker with and redesign as needed.
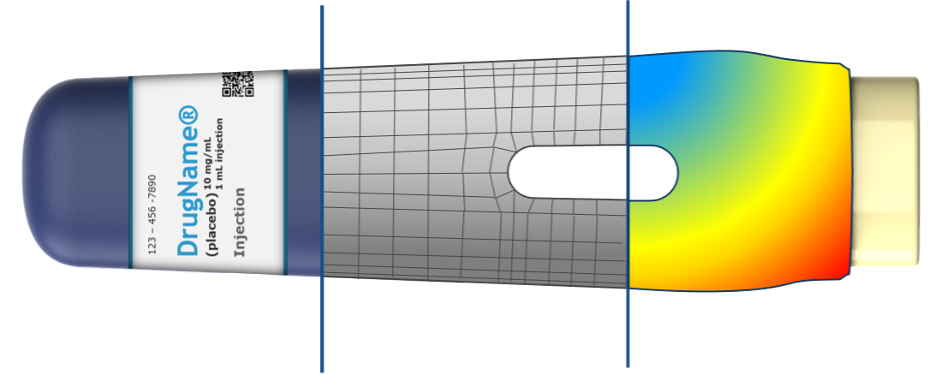
Figure 1: The concept geometry is split into a number of elements (hence the name finite elements). Loads can then be applied, and the model can be solved, giving insights into the product behavior in various loading scenarios.
– “Often when designing a new product from scratch, you’ll rely somewhat on gut feeling. If you need to change the material or dimensions of a physical prototype in a later iteration, the production of the new prototype takes time and costs money. If you simulate the prototype instead, you can e.g. change the material in the simulation and get the result that same day,” explains Alexandre Gegelashvili.
Alexandre Gegelashvili and his colleagues at Technolution A/S work with all aspects of the Finite Element Method for clients in the medical devices industry.
Simulating basic functionality
Take the example of an everyday life product like the screw-top bottle cap shown in figure 1. There are certain requirements to e.g. the amount of torque needed to turn the cap, which is determined by properties like diameter, ramp angle and choice of materials.

Figure 2: Simulating cap screwed onto a bottle
In the same way, a medical device has certain properties that can be simulated with FEM to assess, whether the functional requirements are fulfilled.
– “Maybe the user is an arthritis patient and therefore shouldn’t have to apply a force higher than 10 Newton to activate the drug release button. The simulation makes it possible to assess, whether this requirement is met, thereby reducing the number of iterations necessary to make a working prototype ready for testing ,” says Alexandre Gegelashvili.
At Technolution A/S, the software suite, Ansys Mechanical, is used for static calculations and LS-DYNA for dynamic simulations. For simpler calculations with few components, SOLIDWORKS Simulation and PTC Creo Simulation are used.
From a simulation point of view, the FEM experts at Technolution A/S split the development timeline into six individual simulation areas:
- Early concept selection - enables the design team to make decisions based on data when selecting early concepts.
- Functional assessments - makes it possible to assess e.g. force requirements for button activation.
- Variation studies - reveals the impact on functionality by making small design changes.
- Shelf-life calculations - shows the long-term performance of e.g. a preloaded spring.
- Drop testing - investigates device performance during and after impact.
- Assembly simulations - provides important data for setting up manufacturing equipment.
In the early concept selection stage, the product design uncertainty is high and the level of detail low. Oppositely, at the end of the timeline, where drop testing and assembly simulations reside, the detail level is high and the design uncertainty low. Drop testing, for instance, is a complex simulation that explores, how the device handles impact.
Imagine a medical device dropping onto a concrete floor as shown in figure 2. Can it withstand impact without breaking apart or compromising the functionality?
– “The issue with making real-world drop tests is obviously that you need to have a functional device with all parts assembled – in essence a fully assembled prototype . This is where you can save a lot by simulating the drop test instead,” says Alexandre Gegelashvili.
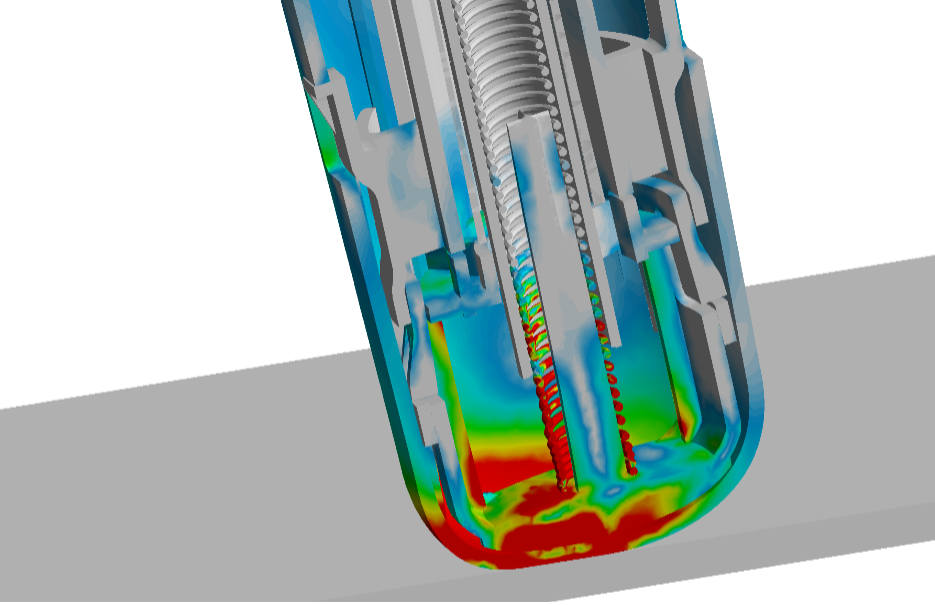
Figure 3: Stresses in our in-house developed autoinjector during the first milliseconds of an impact with the floor
Makes the impossible possible
Another important advantage with simulating drop tests is that observing the hidden internal parts of the device during impact is either very difficult to do or even impossible with a physical device. Likewise, assessing the shelf-life of a device is much easier to do in a simulation, where things like material aging data for polymers makes it possible to simulate e.g. the creep-resistance of a device over a three-year storage period within hours or days.
Overall, besides being an engineering tool, FEM is also very much a project management tool.
– “We make major design decisions based on simulation results,” says Peter Werner Hansen, head of business development at Technolution A/S.
– “It gives you raw data on which to base your decisions. This applies to the early concept selection stage as well as late in development, where simulation makes it possible to test and assess your product in ways not otherwise possible. This is of great value to the management.”
Want to know more?
Insights you might also be interested in:

Why you should apply virtual DoE to the design process
Early product insights and fewer physical tests are some of the benefits of using Design of Experiments in medical device development.