How an FDA pre-submission enhances your usability engineering process

Discover the advantages of incorporating FDA pre-submission insights into your usability engineering process for streamlined success.
Principles for Combination Products: Questions and Answers Guidance for Industry and FDA Staff

news from fda Application of Human Factors Engineering Principles for Combination Products — Questions and Answers Guidance for Industry and FDA Staff. In September, 2023, FDA released their guidance ‘Application of Human Factors Engineering Principles for Combination Products: Questions and Answers’. This guidance finalizes the February 2016 draft version entitled ‘Human Factors Studies and Related […]
When is a new summative evaluation needed?
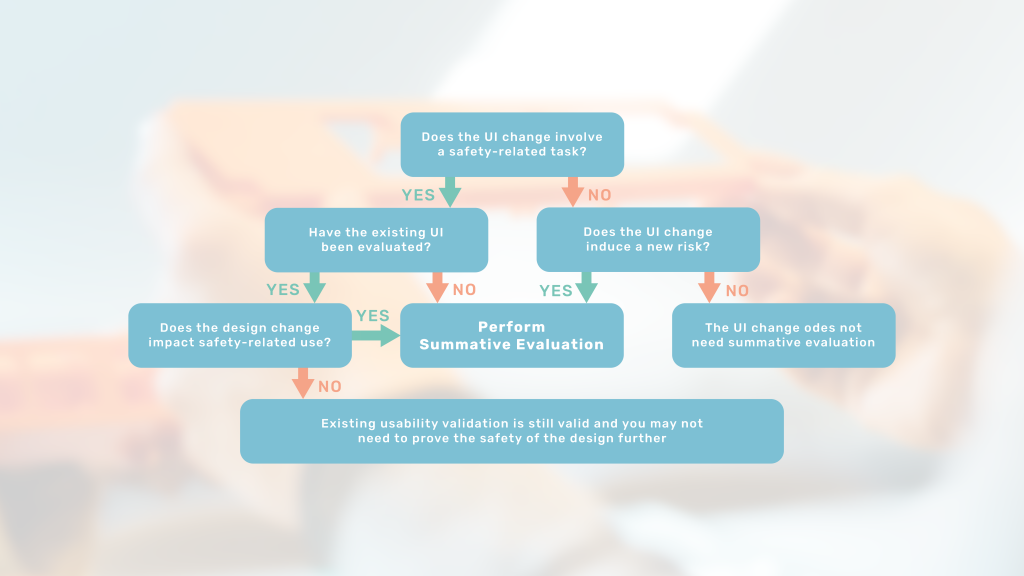
This article will introduce you to assessing the usability impact of your design change and thereby plan for any needed summative evaluation.
Using task analysis to identify potential use errors and get valuable design input

Find out how you can identify use errors and their sources and get valuable design input with PCA analysis.