How an FDA pre-submission enhances your usability engineering process

Discover the advantages of incorporating FDA pre-submission insights into your usability engineering process for streamlined success.
Protected: Successfully designing products
There is no excerpt because this is a protected post.
How not to be part of the 80% of product innovations that fail
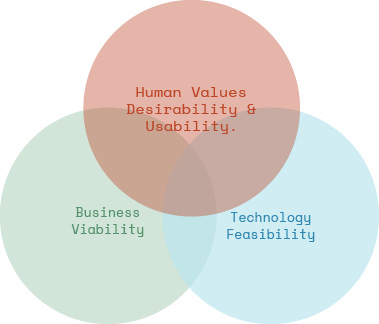
It’s not just about having a great idea but also about balancing desirability, feasibility, and viability.
Data and imagination in innovation

In this article, we’ll explore why imagination is just as important as analytics when it comes to driving innovation.
Principles for Combination Products: Questions and Answers Guidance for Industry and FDA Staff

news from fda Application of Human Factors Engineering Principles for Combination Products — Questions and Answers Guidance for Industry and FDA Staff. In September, 2023, FDA released their guidance ‘Application of Human Factors Engineering Principles for Combination Products: Questions and Answers’. This guidance finalizes the February 2016 draft version entitled ‘Human Factors Studies and Related […]
Bridging drug and device

Read this whitepaper on how to bridge the gap between drug and device on end-to-end device development projects and device platform assessment tasks.
When is a new summative evaluation needed?
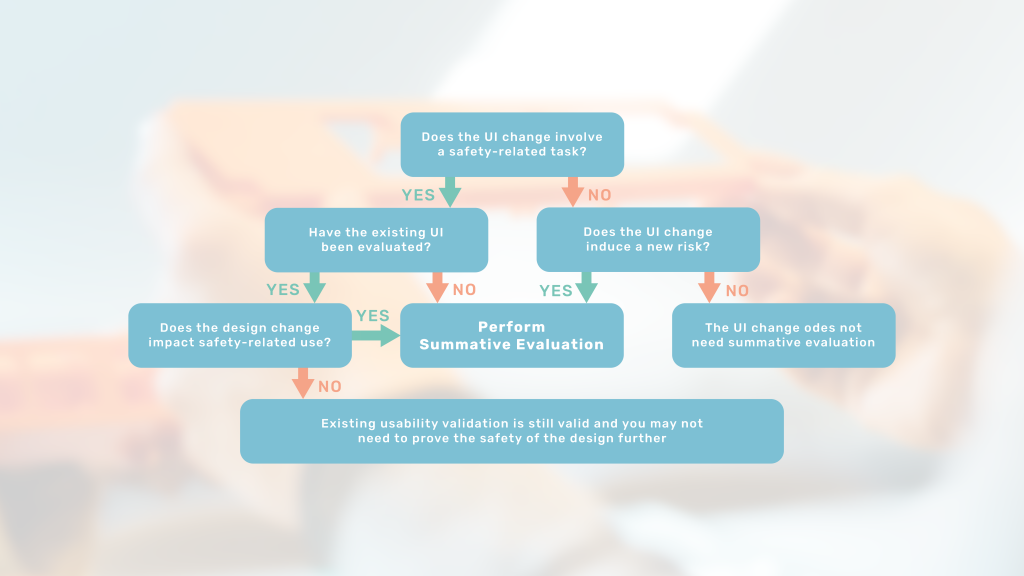
This article will introduce you to assessing the usability impact of your design change and thereby plan for any needed summative evaluation.
Using task analysis to identify potential use errors and get valuable design input

Find out how you can identify use errors and their sources and get valuable design input with PCA analysis.
What are the Benefits of Simulation with the Finite Element Method?

Will a medical device break apart when dropped onto a concrete floor? Does the device remain safe and functional if stored for years on a shelf before reaching the patient?
Why you should apply virtual DoE to the design process

Early product insights and fewer physical tests are some of the benefits of using Design of Experiments in medical device development.